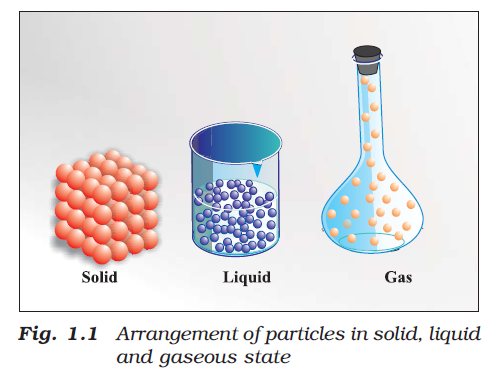
Science can be viewed as a continuing human effort to systematize knowledge for describing and understanding nature.
`=>` Science is sub-divided into various disciplines like chemistry, physics, biology, geology etc. for the sake of convenience.
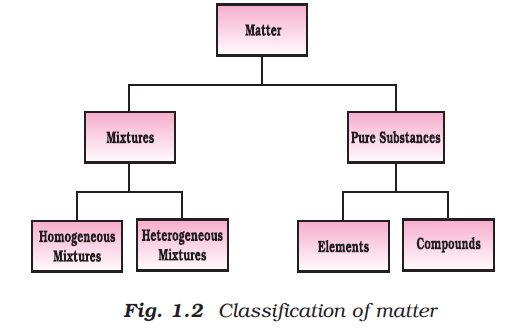
Definition of Chemistry : It is the branch of science that studies the composition, properties and interaction of matter. The aspects of matter can be best described and understood in terms of basic constituents of matter i.e. atoms and molecules.
`text(Note)` : (i) Cis-platin and taxol are effective in cancer therapy.
(ii) AZT (Azidothymidine) used for helping AIDS victims.
`=>` Dry ice along with ether is used as refrigerant. Drugs given to make a patient unconscious before operation are called anesthetics, and drugs used as pain killers are called sedatives.
`=>` Anaemic patients are deficient in iron.
`=>` Fertilisers contain `N`, `P` with `H` as essential constituent. Thus, chlorine is not generally present in fertilizers.
`=>` Bones are considered as reservoir of `Ca`, `Mg` and `PO_4^(3-)` ions. Thus, the main constituent of bones is calcium phosphate.
`=>` The substance present in abundant amounts in sea-water is common salt (`NaCl`) with significant amount of `Mg^(2+)` ions also.
`=>` Many life saving drugs have been isolated from plant and animal sources or prepared by synthetic methods.
`=>` Production of superconducting ceramics, conducting polymers, optical fibres and large scale miniaturization of solid state devices is possible. With a better understanding of chemical principle it has now become possible to design and synthesize new materials having specific magnetic, electric and optical properties.
`=>` CFCs (chlorofluorocarbons) is the environmentally hazardous refrigerants which is responsible for ozone depletion in the stratosphere.
`=>` A gas that cannot be collected over water is `SO_2`.
`=>` The purity of gold is generally expressed as carats. The pure gold is known as 24 carats.
`=>` Vitamin `B_(12)` cyanocobalamine contains cobalt.
`=>` Phenolpthalein gives colour when the solution is alkaline.
`=>` `Ca^(2+)`, `Mg^(2+)` sets of ions associated with hard water.
`=>` Carbogen is a mixture `O_2` and `CO_2`.
`=>` Rubber is made hard and strong by adding sulphur.
`=>` The major constituent of the atmosphere is nitrogen. Our atmosphere contains about `79%` nitrogen.
`=>` Iodine is most soluble in `KI`(aq). `KI +I_2 → KI_3`. In fact `KI` is one of those important compounds which can absorb as well as gives off `I_2` on heating.
`=>` Sodium thiosulphate is used in photography because of its complex forming behaviour. It is used for the removal of undecomposed silver bromide from the film. A complex is formed in this process `[Ag (S_2O_3)_2]^(3-)`.
`=>` Gases exert pressure due to collision of their molecules with the wall of container. The dimensions of pressure are same as that of energy per unit volume.
Science can be viewed as a continuing human effort to systematize knowledge for describing and understanding nature.
`=>` Science is sub-divided into various disciplines like chemistry, physics, biology, geology etc. for the sake of convenience.
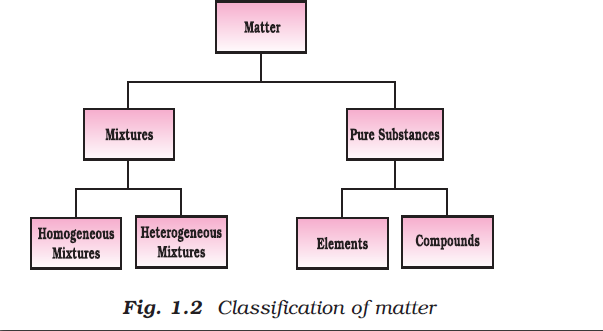
Definition of Chemistry : It is the branch of science that studies the composition, properties and interaction of matter. The aspects of matter can be best described and understood in terms of basic constituents of matter i.e. atoms and molecules.
`text(Note)` : (i) Cis-platin and taxol are effective in cancer therapy.
(ii) AZT (Azidothymidine) used for helping AIDS victims.
`=>` Dry ice along with ether is used as refrigerant. Drugs given to make a patient unconscious before operation are called anesthetics, and drugs used as pain killers are called sedatives.
`=>` Anaemic patients are deficient in iron.
`=>` Fertilisers contain `N`, `P` with `H` as essential constituent. Thus, chlorine is not generally present in fertilizers.
`=>` Bones are considered as reservoir of `Ca`, `Mg` and `PO_4^(3-)` ions. Thus, the main constituent of bones is calcium phosphate.
`=>` The substance present in abundant amounts in sea-water is common salt (`NaCl`) with significant amount of `Mg^(2+)` ions also.
`=>` Many life saving drugs have been isolated from plant and animal sources or prepared by synthetic methods.
`=>` Production of superconducting ceramics, conducting polymers, optical fibres and large scale miniaturization of solid state devices is possible. With a better understanding of chemical principle it has now become possible to design and synthesize new materials having specific magnetic, electric and optical properties.
`=>` CFCs (chlorofluorocarbons) is the environmentally hazardous refrigerants which is responsible for ozone depletion in the stratosphere.
`=>` A gas that cannot be collected over water is `SO_2`.
`=>` The purity of gold is generally expressed as carats. The pure gold is known as 24 carats.
`=>` Vitamin `B_(12)` cyanocobalamine contains cobalt.
`=>` Phenolpthalein gives colour when the solution is alkaline.
`=>` `Ca^(2+)`, `Mg^(2+)` sets of ions associated with hard water.
`=>` Carbogen is a mixture `O_2` and `CO_2`.
`=>` Rubber is made hard and strong by adding sulphur.
`=>` The major constituent of the atmosphere is nitrogen. Our atmosphere contains about `79%` nitrogen.
`=>` Iodine is most soluble in `KI`(aq). `KI +I_2 → KI_3`. In fact `KI` is one of those important compounds which can absorb as well as gives off `I_2` on heating.
`=>` Sodium thiosulphate is used in photography because of its complex forming behaviour. It is used for the removal of undecomposed silver bromide from the film. A complex is formed in this process `[Ag (S_2O_3)_2]^(3-)`.
`=>` Gases exert pressure due to collision of their molecules with the wall of container. The dimensions of pressure are same as that of energy per unit volume.